Interatomic van der Waals forces
The Lennard Jones Potential is common potential used to model VanderWaals interactions which is expressed as follows,
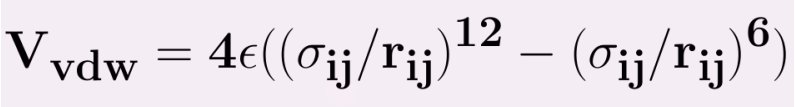
ε = the minimum (well depth) of the potential for the interaction between atom i and j.
σij = the collision diameter,at which inter particle potential is zero.
rij = the distance between the particles.
The parameters ε,σ is not given for a specific pair and can be found by using Lorentz-berthelot rules,
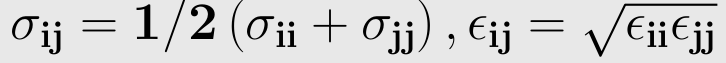
The term r-12 is called repulsive term, observed at short ranges due to overlapping of electron orbitals. The term r-6 describes the attraction at long ranges.
